Exploring Polycystic Kidney Disease: Genetics and Management Insights
Written on
Introduction to Polycystic Kidney Disease
Polycystic Kidney Disease (PKD) encompasses a variety of genetic disorders marked by the development of multiple cysts within the kidneys, which can result in serious complications, including renal failure. Gaining insight into the genetic and molecular frameworks of PKD is essential for creating targeted therapeutic strategies and effectively managing the physiological challenges associated with the disease (Chapman & McGill, 2021).
Genetic Foundations of Polycystic Kidney Disease
The most prevalent variant of PKD is autosomal dominant polycystic kidney disease (ADPKD), which follows an autosomal dominant inheritance mode. This indicates that inheriting just one mutated gene from a parent is sufficient to trigger the disease. The two primary genes linked to ADPKD are PKD1 and PKD2 (Harris & Torres, 2009). Mutations in PKD1, located on chromosome 16, account for approximately 85% of ADPKD instances, while mutations in PKD2, found on chromosome 4, make up the remaining 15% (Harris & Torres, 2009).
Both PKD1 and PKD2 produce proteins that are crucial for maintaining cell structure, adhesion, and signaling. The PKD1 protein, known as polycystin-1, acts as a receptor on the cell surface, while PKD2, referred to as polycystin-2, functions as a calcium channel (Harris & Torres, 2009). These proteins work in unison to regulate cell growth, differentiation, and adhesion. When mutations occur in either gene, this balance is disrupted, leading to uncontrolled cell growth and cyst formation (Harris & Torres, 2009).
While ADPKD is the most common form, other genetic mutations can also lead to PKD. Autosomal recessive polycystic kidney disease (ARPKD) is caused by mutations in both copies of the PKHD1 gene, usually passed down from unaffected carriers. Additionally, rarer types of PKD can be linked to mutations in other genes, such as GANAB and HNF1B (Harris & Torres, 2009).
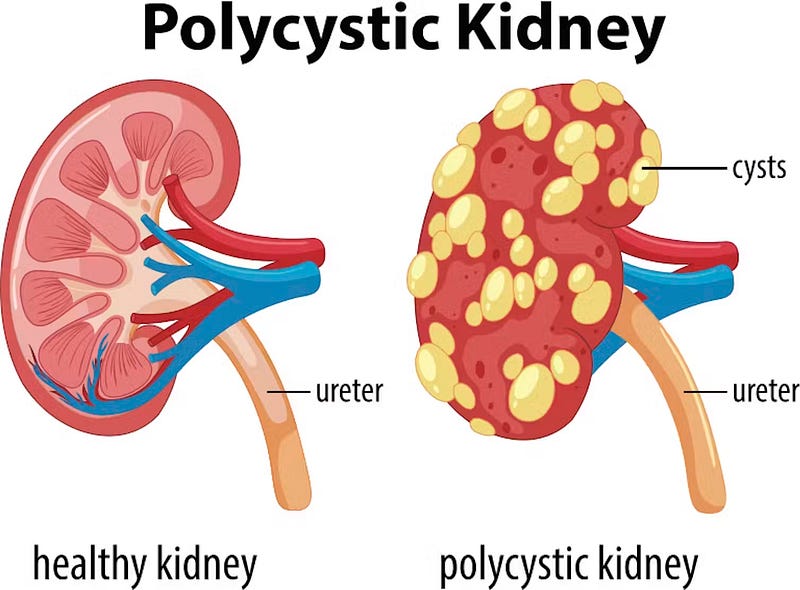
Molecular Mechanisms Behind the Disease
ADPKD and ARPKD stem from mutations in specific genes, leading to unique molecular issues. In ADPKD, the problematic genes are PKD1 and PKD2. PKD1 encodes polycystin-1, functioning as a cellular antenna, while PKD2 encodes polycystin-2, acting as a gatekeeper for channels (Halvorson et al., 2010). Together, they regulate vital processes like cell growth, adhesion, and signaling. Mutations in either PKD1 or PKD2 disrupt this essential partnership, impairing polycystin-1's ability to respond to external signals and compromising polycystin-2's channeling function (Halvorson et al., 2010). This results in disrupted calcium signaling within the cells, which is crucial for maintaining normal cellular functions. Consequently, cells lining the kidney tubules lose growth control, leading to abnormal proliferation and cyst formation (Halvorson et al., 2010).
ARPKD, while less common, has a different genetic basis. Mutations in the PKHD1 gene, which encodes fibrocystin/polyductin, are responsible (Halvorson et al., 2010). This protein is located in primary cilia, tiny hair-like structures on cell surfaces that play critical roles in cell signaling and development. In ARPKD, defective fibrocystin/polyductin impairs primary cilia function, leading to abnormal signaling and cyst formation within kidney collecting ducts (Halvorson et al., 2010).
Although genetic mutations initiate the molecular disruption, the effects are extensive. Impaired calcium signaling and dysfunctional primary cilia trigger a series of consequences, including altered cell adhesion, increased proliferation, and inflammation, all contributing to the progressive growth of cysts and the eventual compromise of kidney function (Halvorson et al., 2010).
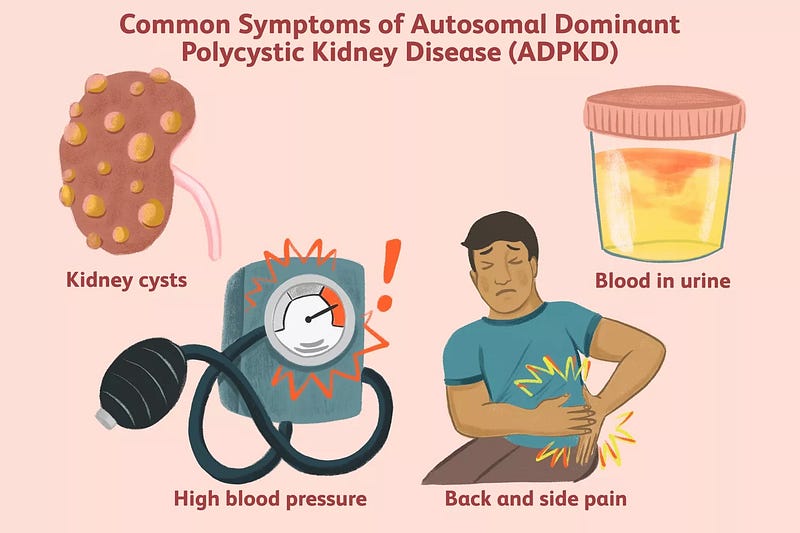
Physiological Consequences of PKD
The primary impact of PKD is on kidney function. As cysts grow larger, they gradually replace healthy tissue, impairing the kidneys’ ability to filter waste and regulate fluid balance. This deterioration can lead to chronic kidney disease, which may necessitate dialysis or kidney transplants. The growth of cysts, coupled with inflammation, also contributes to hypertension, creating a harmful cycle (National Institute of Diabetes and Digestive and Kidney Diseases, n.d.).
As kidney function declines, the body struggles to remove waste products and manage electrolyte levels, potentially resulting in imbalances in potassium, calcium, and phosphorus. These imbalances may require dietary changes and medications to restore equilibrium (National Institute of Diabetes and Digestive and Kidney Diseases, n.d.).
Cysts may also develop in the liver, pancreas, and other organs, affecting their functionalities. Liver cysts can disrupt digestion, blood clotting, and bile production, while pancreatic cysts can interfere with hormone production and blood sugar control (National Institute of Diabetes and Digestive and Kidney Diseases, n.d.). The presence of numerous cysts can lead to significant discomfort, particularly in the flanks and abdomen. Pain may be constant or intermittent, varying in intensity based on cyst size and location, and ruptured cysts can trigger sudden, severe pain (National Institute of Diabetes and Digestive and Kidney Diseases, n.d.).
Women with PKD face heightened risks during pregnancy, including preeclampsia and premature birth, along with potential fertility issues stemming from hormonal imbalances or physical blockages caused by cysts (National Institute of Diabetes and Digestive and Kidney Diseases, n.d.).
Current Approaches to Treatment
While there is no definitive cure for PKD, several treatment options exist to manage symptoms and associated complications. A key treatment strategy involves controlling hypertension, a common complication linked to the disease. Medications such as angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs) are often prescribed to help regulate blood pressure (Halvorson et al., 2010). Effective blood pressure management is vital, as it can slow the progression of kidney damage.
Pain management is another crucial aspect of PKD treatment. Over-the-counter pain relievers may provide relief, but it’s essential to consult with healthcare providers, as some medications could further compromise kidney function. In severe cases, surgical procedures may be required to drain painful cysts or address bleeding or infection (Halvorson et al., 2010).
A notable advancement in PKD treatment is the use of tolvaptan, a medication that has demonstrated effectiveness in slowing the increase in kidney volume and the decline in function for patients with PKD, representing a significant step forward in disease management (Torres et al., 2012).
In addition to medical treatments, lifestyle changes such as maintaining a balanced diet, engaging in regular physical activity, and avoiding smoking play a vital role in managing PKD. These modifications can help control blood pressure, lower the risk of cardiovascular disease, and promote overall kidney health (National Institute of Diabetes and Digestive and Kidney Diseases, n.d.).
In this video titled "Polycystic kidney disease - causes, symptoms, diagnosis, treatment, pathology," viewers will gain insights into the underlying causes of PKD, explore its symptoms, and understand the various treatment options available.
The video "Polycystic Kidney Disease, Causes, Signs and Symptoms, Diagnosis and Treatment" provides a comprehensive overview of PKD, discussing its causes, signs, and effective treatment strategies.
Enjoyed the read?
Here are some ways you can show your support. It would mean a lot to me!? Applaud The Story | ? Comment Your Insights | ? Follow For Updates ? Read More Stories | ? Share It With Friends | ? Buy Me An Iced Coffee ?? Disclaimer & Disclosures | ? This article may use affiliate links which may earn a commission for purchases made at no additional cost to you.