Gene Therapy: A New Hope for Children with SCID
Written on
Chapter 1: Understanding SCID
Severe Combined Immunodeficiency (SCID) was once considered a fatal diagnosis. Thankfully, advancements in gene therapy are now enabling some affected children to lead fulfilling lives.

Deoxyadenosine: The Danger Within
Deoxyadenosine is a byproduct formed during the breakdown of nucleic acids in our cells. While your body is continuously producing it, this molecule is highly toxic to immune cells. If not broken down swiftly, it can lead to the demise of these cells, leaving the body susceptible to infections.
Fortunately, most individuals possess a gene that encodes for a protein called adenosine deaminase, which helps convert deoxyadenosine into less harmful substances. However, approximately 1 in 100,000 people are born with mutations in their ADA gene, resulting in a dangerous accumulation of deoxyadenosine that can hinder the development of the immune system. This condition, known as ADA-SCID, if untreated, can lead to the death of most affected children by the age of two.
Historically, SCID has conjured images of the “bubble boy,” but today, children with ADA-SCID no longer need to live in total isolation. The standard treatment is now a stem cell transplant, preferably from a matched sibling. However, only 20% of children with ADA-SCID find a suitable donor, and even matched transplants necessitate lifelong immunosuppression. While bi-weekly pegylated-ADA injections serve as a temporary solution, they are not as effective as transplants and can be excessively costly.
Enter Gene Therapy
Gene therapy for ADA-SCID began in the mid-90s but initially yielded mediocre results; the modified cells did not survive long enough to offer substantial protection. In 2009, a breakthrough was reported in the New England Journal of Medicine, highlighting a significant improvement.

The study indicated successful restoration of ADA levels in 8 out of 10 participants. While this was a monumental advancement, a patient later developed T-cell leukemia, possibly due to insertional oncogenesis — where the introduced gene integrates into the genome in a manner that encourages cancer growth.
A recent report in the New England Journal signifies that gene therapy for ADA-SCID may be ready for widespread application.

In this latest study, 50 children from the U.S. and Europe underwent gene therapy for ADA-SCID. A crucial innovation was the use of a lentiviral vector, which is expected to carry a lower risk of cancer. Remarkably, of the 50 children treated, 48 have shown signs of being effectively cured.
How the Process Works
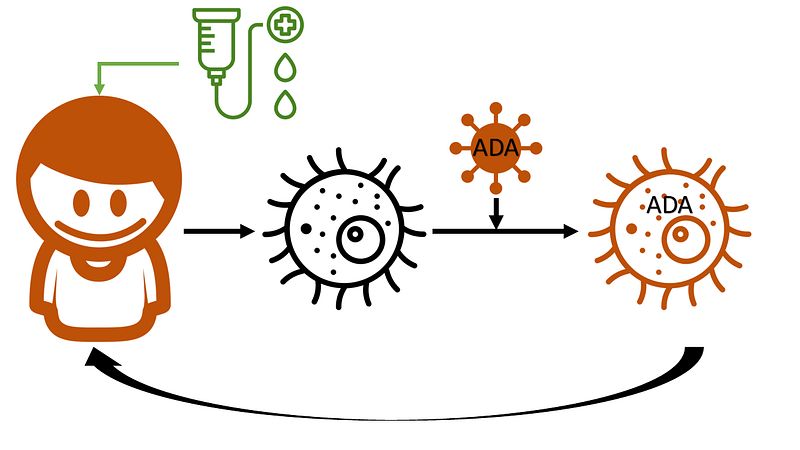
The gene therapy process begins with the extraction of stem cells from either the bone marrow or peripheral blood. Outside the body, these cells are exposed to a modified lentiviral vector containing the ADA gene in RNA form. This virus has been engineered to prevent replication while still providing the necessary tools to convert the RNA into complementary DNA and integrate it into the host cell's nuclear DNA.
After the modified stem cells are evaluated for safety and viability, they are prepared for reinfusion. Meanwhile, the patient receives busulfan to reduce the number of existing wild-type stem cells in the bone marrow, creating space for the modified cells to integrate.
Following successful reinfusion, these modified cells ideally settle in the bone marrow, proliferate, and generate immune cells capable of metabolizing deoxyadenosine. Out of the 50 children, two did not achieve successful engraftment and returned to enzyme replacement therapy, while one found a donor for a stem cell transplant.
Focusing on the 48 who did experience successful engraftment, the average age of participants was under one year. Their pre-treatment ADA levels were nearly nonexistent, but post-treatment, these levels surged significantly.
The immune system exhibited a remarkable resurgence, with diverse immune cell types thriving. The toxic deoxyadenosine levels dropped dramatically, and genetic markers associated with the new ADA gene were found in various white blood cells, including granulocytes, T-cells, and B-cells. Overall, white blood cell and immunoglobulin counts returned to normal ranges. None of the 48 children required further ADA injections or immunosuppressive medications.
While this study may not directly impact everyday practices for most healthcare providers, it serves as a reminder of the incredible strides science has made in alleviating human suffering.
As of this writing, after two to three years of follow-up, all 50 children remain alive.
Evaluating Risks and Future Outlook
No treatment is without its challenges. Fifteen children experienced severe infections post-gene therapy, potentially linked to the conditioning regimen involving bone marrow. Other adverse effects included temporary immune reconstitution syndrome in two children, along with common gastrointestinal issues and fever.
However, encouragingly, none of the children have developed cancers, autoimmune disorders, or replication-competent lentivirus — at least for now. Given that only a few years have passed since treatment, the long-term sustainability of these results remains uncertain. Continuous monitoring will be essential for these children throughout their lives.
Despite these uncertainties, it’s difficult to view this as anything other than a remarkable success. This therapy offers these children a chance at a normal life, something that would have seemed unattainable just two decades ago.
A version of this commentary originally appeared on medscape.com.